You are here
Clinical trials
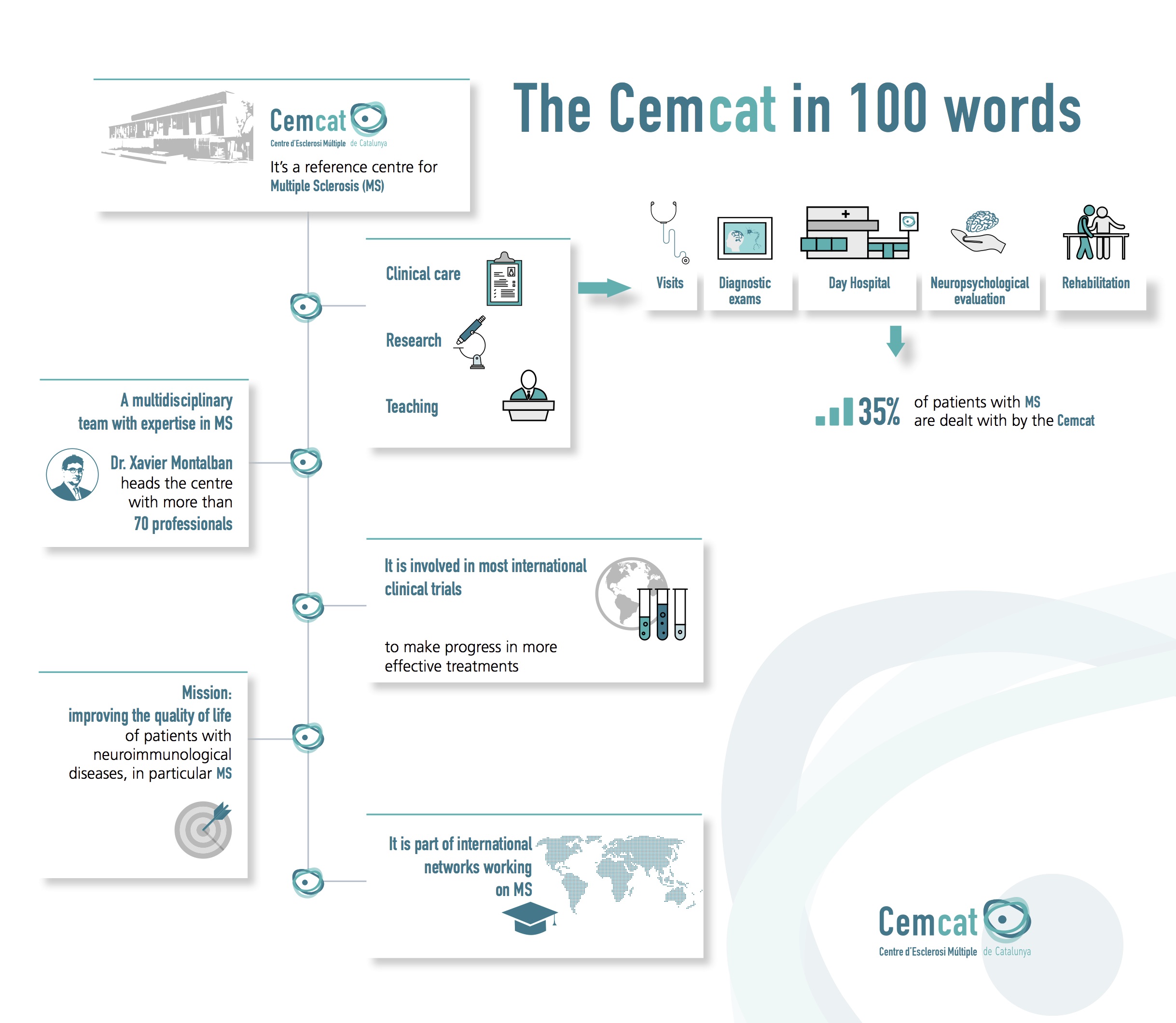
Cemcat takes part in most international clinical trials contributing to the approval of drugs used in the treatment of multiple sclerosis. In this regard, Cemcat is firmly committed to high-level transfer research allowing for progress in new and more effective drugs and treatments.
Clinical trials are one of the main research indicators at Cemcat. There are currently around 20 clinical trials in progress involving more than 120 patients.
From 2017 to date, the European Medicines Agency (EMA) has authorized the marketing of up to six new drugs for the treatment of multiple sclerosis (Mavenclad® in 2017, Ovecrus® in 2018, Mayzent® and Zeposia® in 2020 and Kesimpta® and Ponvory® in 2021). Cemcat was involved in the clinical trials of all six of these new drugs. This also means that a number of patients were able to benefit from the products before they were commercially released, while knowledge was built up about their early management.
We would particularly emphasise the scientific relevance of the clinical trials in which Cemcat takes part: over recent years six of these clinical trials have been published in the Lancet Neurology, four in the Multiple Sclerosis Journal, three in the New England Journal of Medicine, two in Multiple Sclerosis and Related Disorders, in Neurology, and in the Journal of Neurology. Trials have also been published in the Annals of Neurology, the European Journal of Neurology, Frontiers in Immunology, JAMA, the Lancet, and in Neurology Neuroimmunology & Neuroinflammation.
An average of 10 Cemcat professionals take part in each clinical trial. This includes neurologists at the centre, nurses, data managers, etc. On occasion, the dynamic of a clinical trial has also led to changes to the dynamic of the centre itself in terms of working hours, staff allocation, etc. In such cases Cemcat adapts its functioning in order to allow the clinical trial to be staged.
For Cemcat it is vital to maintain a high level of quality in its participation in studies so as to remain a pioneering centre in clinical trials involving multiple sclerosis.