You are here
Clinical Trials
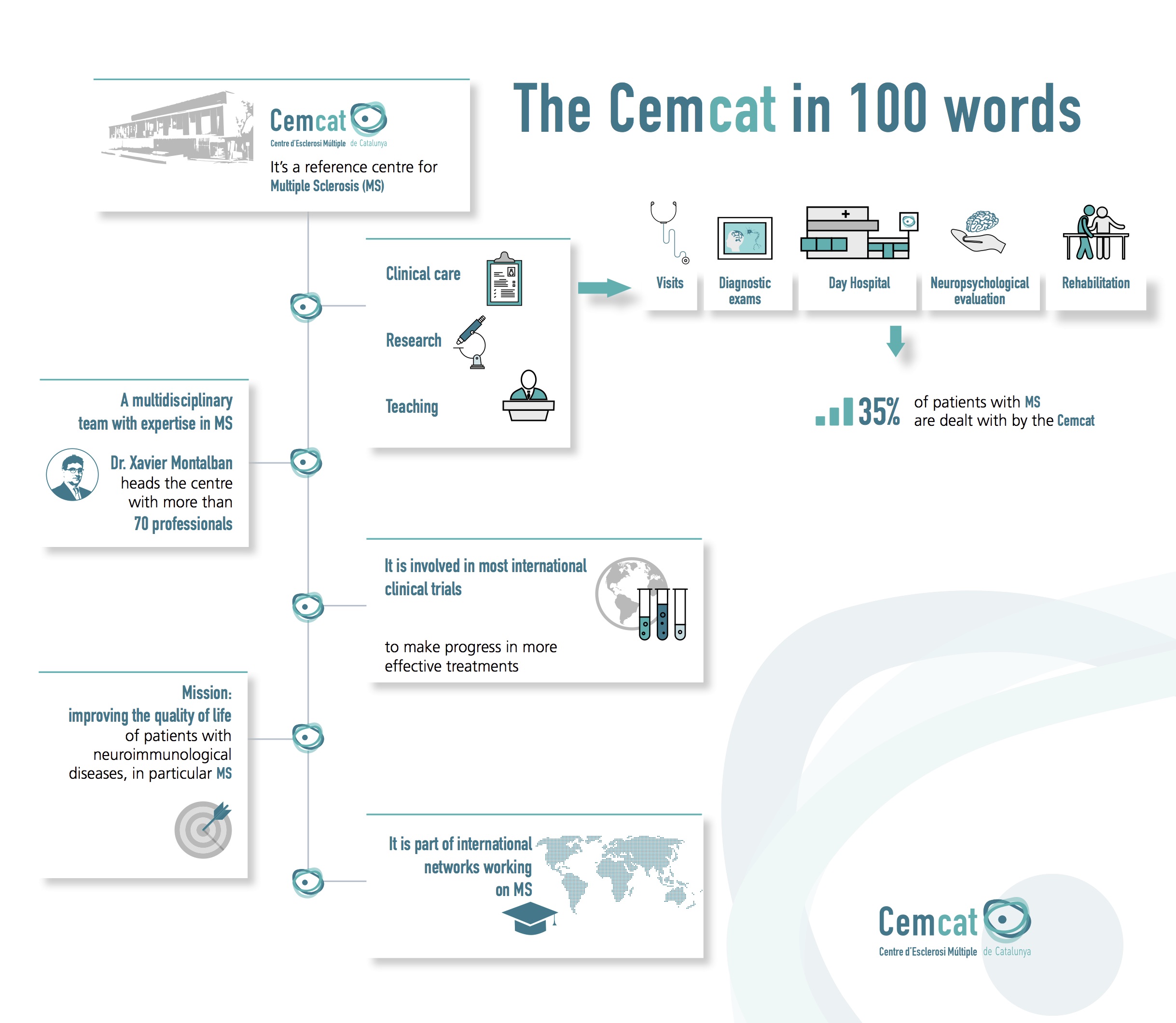
Clinical trials comprise one of the most important aspects of the fight against multiple sclerosis. They are essential with a view to research into new drugs to treat the disease.
Without the involvement of people with multiple sclerosis in clinical trials, it would be impossible to develop new and better solutions to combat the disease. Meanwhile, in some cases patients may benefit from the drugs before they become commercially available.
Trials help determine whether treatments and other interventions are safe and effective against the disease. The studies include patients with a particular diagnosis, and are monitored to guarantee the rights and safety of all participants. It should be emphasised that the Clinical Research Ethics Committee (CEIC) of the Vall d'Hebron Hospital evaluates any clinical trials that are to be undertaken.
Cemcat takes part in most international clinical trials contributing to the approval of drugs used in the treatment of multiple sclerosis.
There are currently around 20 clinical trials in progress involving more than 120 patients.