You are here
Dr. Montalban led a study that proves the efficacy of a drug in primary progressive multiple sclerosis
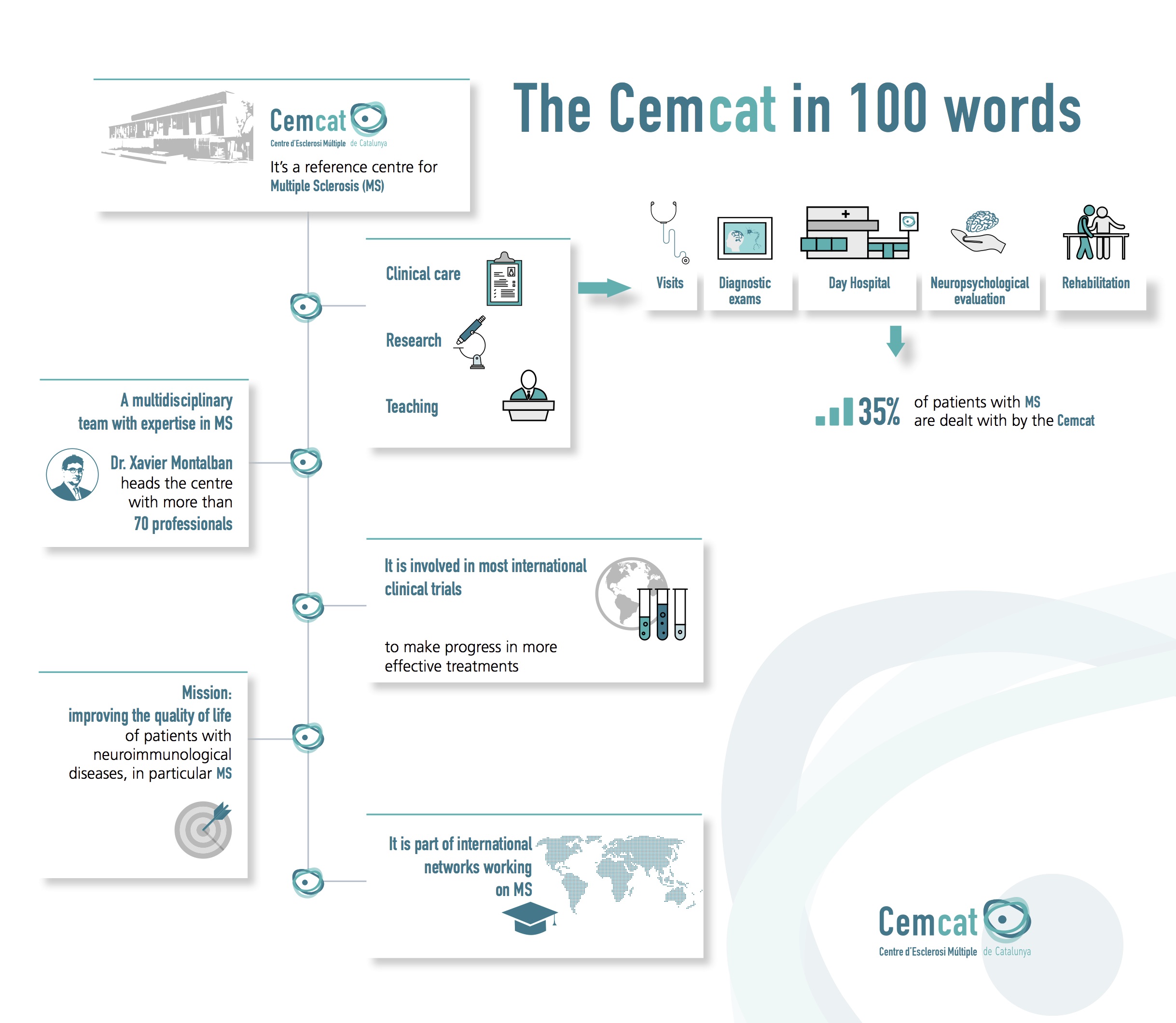
The New Journal of Medicine has published the positive results of Ocrelizumab in the treatment of primary progressive multiple sclerosis (PPMS). Dr. Xavier Montalban, head of the Service of Neuroimmunology of Vall d’Hebron and director of Cemcat, is the corresponding author of this article and leads the Scientific Board of the phase III clinical trial ORATORIO that has risen out these results. The Sclerosis Multiple Centre of Catalonia (known as Cemcat in Catalan) has participated in this clinical trial. The research of this experimental therapy with Ocrelizumab had positive results in phase II, also with the participation of Cemcat.
“For more than 20 years different molecules have been studied but we have now for the first time positive results with a drug to treat primary progressive multiple sclerosis. An important advance that has the potential to find a treatment for these patients, one of the great deals in multiple sclerosis” , comments Dr. Xavier Montalban, who is also the director of the Neuroimmunology group of Vall d’Hebron Institute of Research (VHIR).
The clinical trial ORATORIO, with 732 patients, concludes that Ocrevus (Roche) was able to modify the progression of the disability caused by the disease. In particular, it has shown a reduction of a 24% of the progression and a positive effect in the loss of cerebral volume.
The Sclerosis Multiple Centre of Catalonia (Cemcat) participates in the great majority of international clinical trials that contribute to the approval of drugs used for the treatment of multiple sclerosis. The Cemcat has 25 clinical trials ongoing currently. The trials include the study of therapies to treat the predominant form of the disease, the relapsing-remitting multiple sclerosis, and the progressive forms. About 10 professionals participate in each clinical trial, among which there are researchers, neurologists, neuroradiologists, nurses and data managers.
Multiple sclerosis may be a serious and progressive disease and can cause disability. At Sclerosis Multiple Centre of Catalonia, within a reference hospital in the treatment of the disease, as it is Vall d’Hebron University Hospital, the patient receives a complete and specialized care and a high quality research and teaching is performed.
“Ocrelizumab Versus Placebo in Primary Progressive Multiple Sclerosis” (New England Journal of Medicine, 12/21/2016)